As with other immunoassays , sample preparation for Western Blot (WB) is a crucial step in obtaining optimal results in our experiments.
This week we bring you a brief guide that we hope will help you in preparing samples for Western Blot , from the extraction of the protein to preparing it for loading on the gel.
Western Blot Sample Preparation Guide
1.- Selection Of The Lysate Buffer
The different lysate buffers show differences in their ability to solubilize proteins. Those that contain SDS or other ionic detergents will provide the highest solubility, but in return we run the risk of the protein being denatured.
If the antibody that we are going to use in the immunoassay does not recognize the denatured protein, we should choose a buffer with or even non-ionic detergents, even if we obtain a lower yield.
On the other hand, the location of the protein is another of the determining factors when selecting the lysate buffer. For example, the use of buffers with detergent will be essential in the case of membrane proteins or proteins associated with the cytoskeleton.

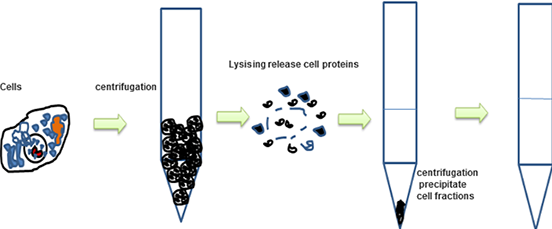
2.- Preparation Of The Lysate
From cell culture
- Let the cells grow until reaching a confluence of 80%.
- Cold wash cells with PBS.
- Aspirate the PBS and add the previously cooled lysate buffer.
- Scrape off adherent cells or digest them with trypsin and transfer the cell suspension to a previously cooled microcentrifuge tube.
- Centrifuge at 4 ° C taking into account that the centrifugation force and the time will vary depending on the cell type.
- Place the tubes on ice and collect the supernatant that will be collected in new tubes previously cooled, discarding the pellet.
From tissue
- Place the tissue in saline at 4ºC and section.
- Place the tissue in Eppendorf tubes and cryogenize in liquid nitrogen.
- Add the lysate buffer and homogenize.
- Centrifuge at 4ºC taking into account that the centrifugation force and the time will vary depending on the type of sample.
- Place the tubes on ice and collect the supernatant that will be collected in new tubes previously cooled, discarding the pellet.
3.- Determination Of Protein Concentration
Protein concentration can be performed by Bradford Assay, Lowry Assay or BCA Assay, bovine serum albumin (BSA) can be used as standard. Once the protein is quantified, it can be loaded onto the gel or frozen and stored for later use.
4.- Preparation Of The Sample For Gel Loading
- Denatured proteins
Usually, in order to make the antibody easier to access the epitope it recognizes, the protein is usually denatured .
To do this, you can use a loading buffer containing the SDS anionic detergent and then boil the mixture at 100ºC for 5 minutes.
In the case of proteins with several transmembrane domains, it can be heated at 70ºC for 10 minutes to avoid the formation of aggregates that would hinder the passage through the gel.
- Native proteins
In certain cases, the antibody does not recognize a linear epitope on the protein, but a conformational epitope made up of a series of non-contiguous amino acids that are close to each other after the three-dimensional folding of the protein.
In these cases, the Western Blot must be carried out under non-denaturing conditions, which basically means avoiding the use of SDS in both the sample and the migration buffers, and avoiding heating the samples.
Some antibodies, moreover, only recognize the protein in its non-reduced form. In these cases it will be necessary to avoid the use of reducing agents such as beta-mercaptoethanol or DDT in loading and migration buffers.